
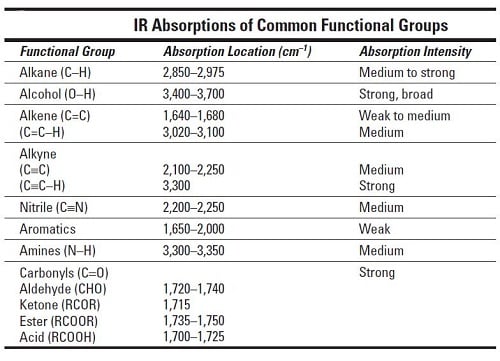
The advantages of infrared spectroscopy are that it is fast and relatively cheap and usually easily accessible in an undergraduate lab. Most of the information that is used to interpret an IR spectrum is obtained from the functional group region. NaCl starts to absorb at about 700 cm-1 and KBr at about 400 cm-1 NaCl and KBr are used because they are ionic crystals and due to their structure they will not absorb in the region you are looking at for organics.

The trend in increasing bond strength, shorter bond, and thereby increasing Relationship between mass of the atom and the frequency of the vibration. Lighter atoms showĪbsorption bands at larger wavenumber such as C-H bond is found betweenģ300-2700 cm-1, C-O (1100 cm-1) and C-Cl (700 cm-1). Required to stretch it (analog to tighter spring). The stronger the bond, the greater the energy To stretch a bond depends on the strength of the bond and the masses Higher frequency (~2100 cm-1) than does a double bond (~ 1650 cm-1),Īs observed in the following diagram. Infrared (IR) spectroscopy is a very useful method for detecting the characteristic bonds of many functional groups through their absorption of infrared light. Is stronger than a double bond, so a triple bond stretches at a More energy is required to stretch a bondĪbsorption bands for stretching vibrations areįound in the functional group region (4000 -14000 cm-1) whereas,Ībsorption bands for bending vibrations are typically found in theįingerprint region (1400 -600 cm-1). (produced by a change of bond length) or bending (resulted inĬhange in bond angle). IR radiation is absorbed by organic molecules andĬonverted into energy of molecular vibration, either stretching The fingerprint region is unique for a molecule and theįunctional group region is similar for molecules with the sameįunctional groups. Percent transmittance or absorbance (Y-axis). With a longer wavelength and lower frequency than visible light.Īn IR spectrum is a plot of wave-number or wavelength (X-axis) vs. IR spectroscopyĬomprises the region of the electromagnetic spectrum that is light
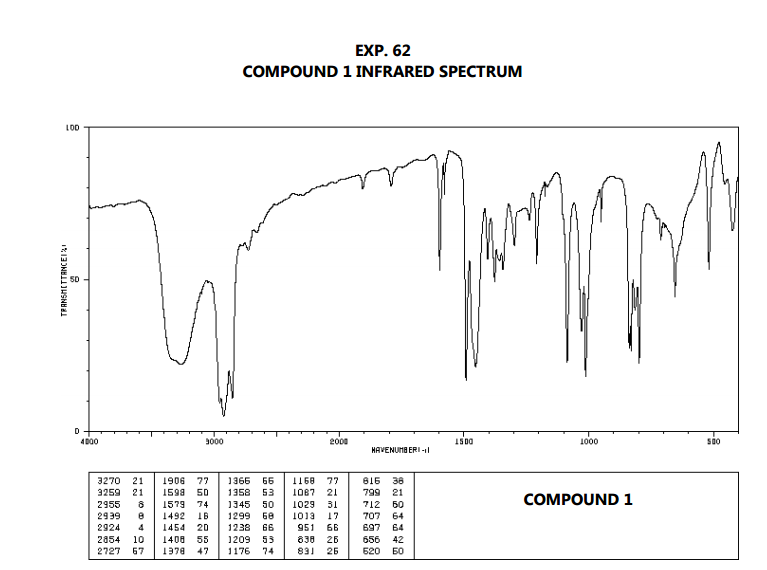
Techniques used by organic and inorganic chemists. We present the first implementation of ML using image-based CNNs for predicting functional groups from a spectroscopic method.Infrared (IR) spectroscopy is one of the most common spectroscopic
#Ir spectrum functional groups full#
Our approach was done such that we have broad functional group models that inference in tandem to provide full interpretation of a spectrum. These models serve to expand the application of FTIR measurements for facile analysis of organic samples. We successfully train models for 15 of the most common organic functional groups, which we then determine via identification from previously untrained spectra. Through web scraping, we acquire intensity-frequency data from 8728 gas phase organic molecules within the NIST spectral database and transform the data into images. The ML models will reduce the amount of time required to analyze functional groups and facilitate interpretation of FTIR spectra. We develop a generalizable model via a machine learning (ML) algorithm using Convolutional Neural Networks (CNNs) to identify the presence of functional groups in gas phase FTIR spectra. Spectral interpretation is a time-consuming process, but it yields important information about functional groups present in compounds and in complex substances. Fourier Transform Infrared Spectroscopy (FTIR) is a ubiquitous spectroscopic technique.


 0 kommentar(er)
0 kommentar(er)
